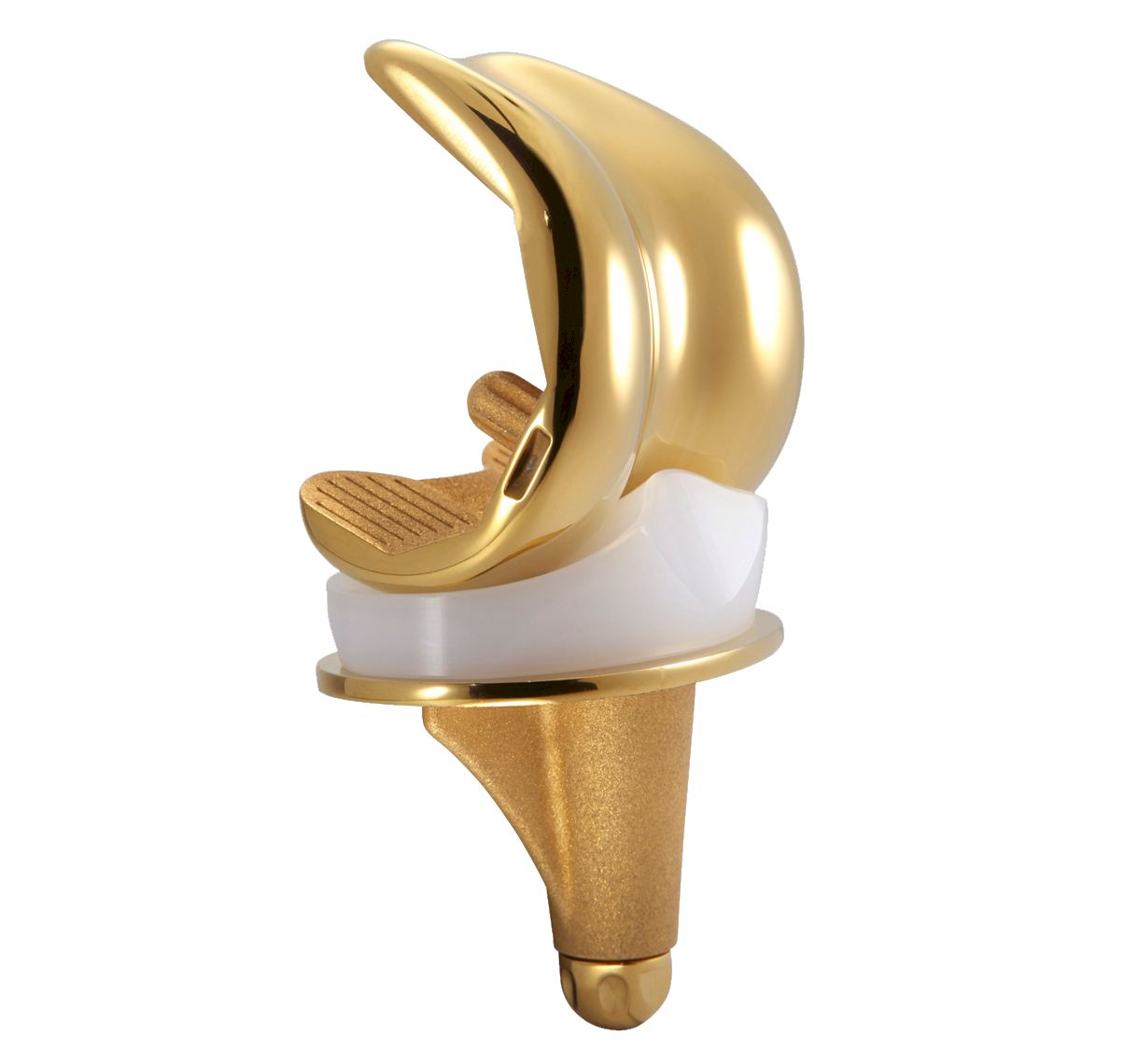
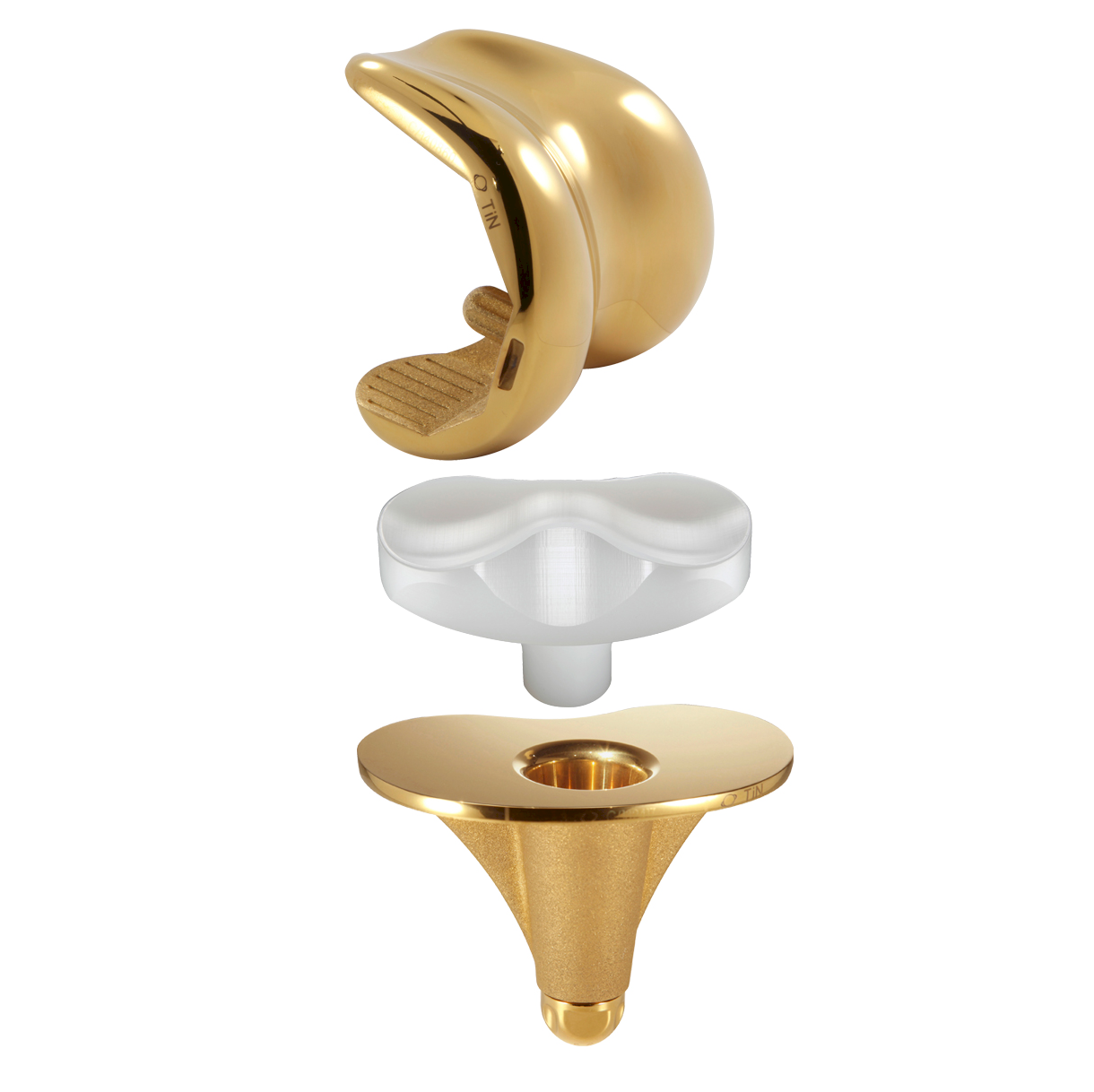



SCORE AS
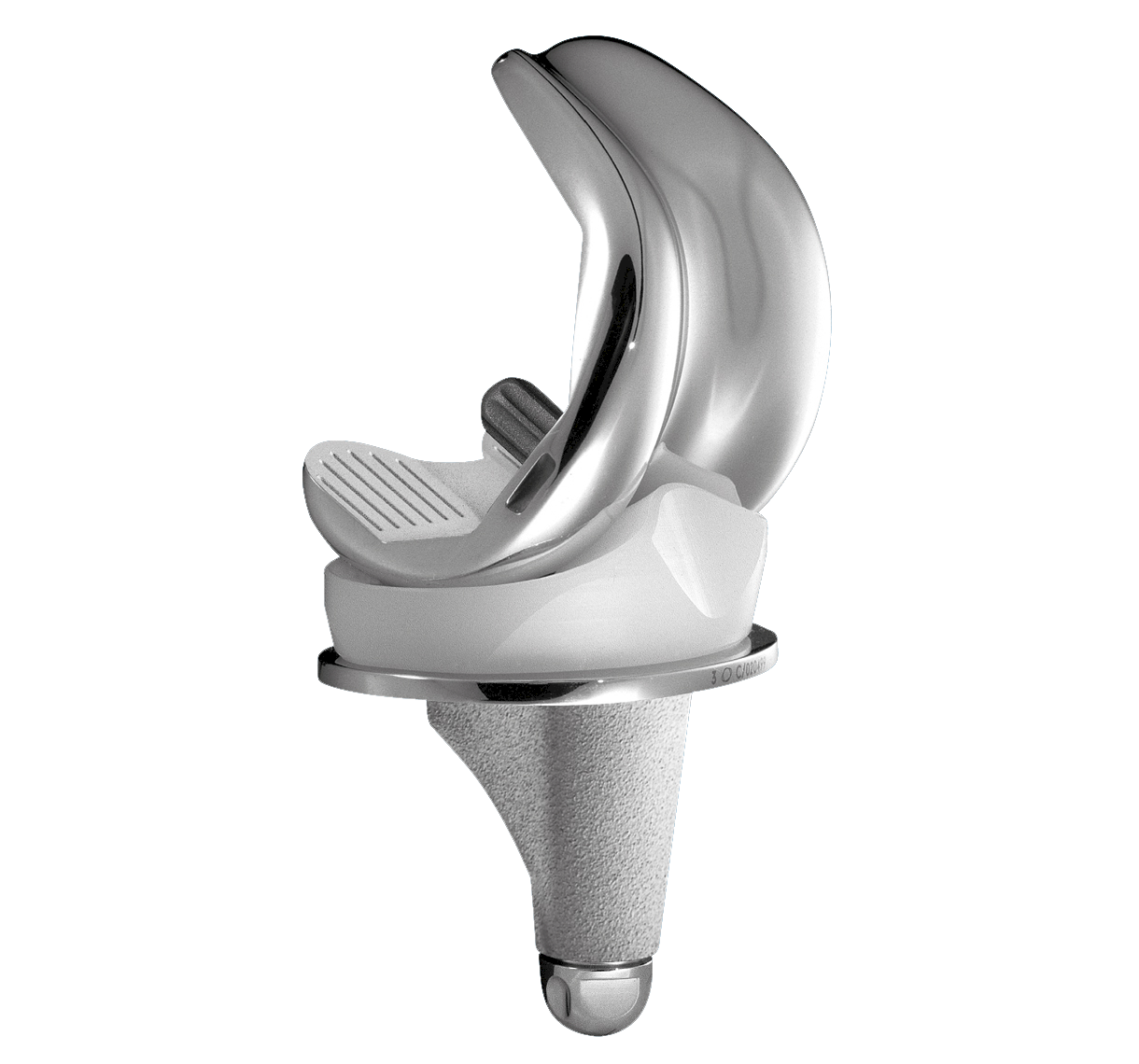
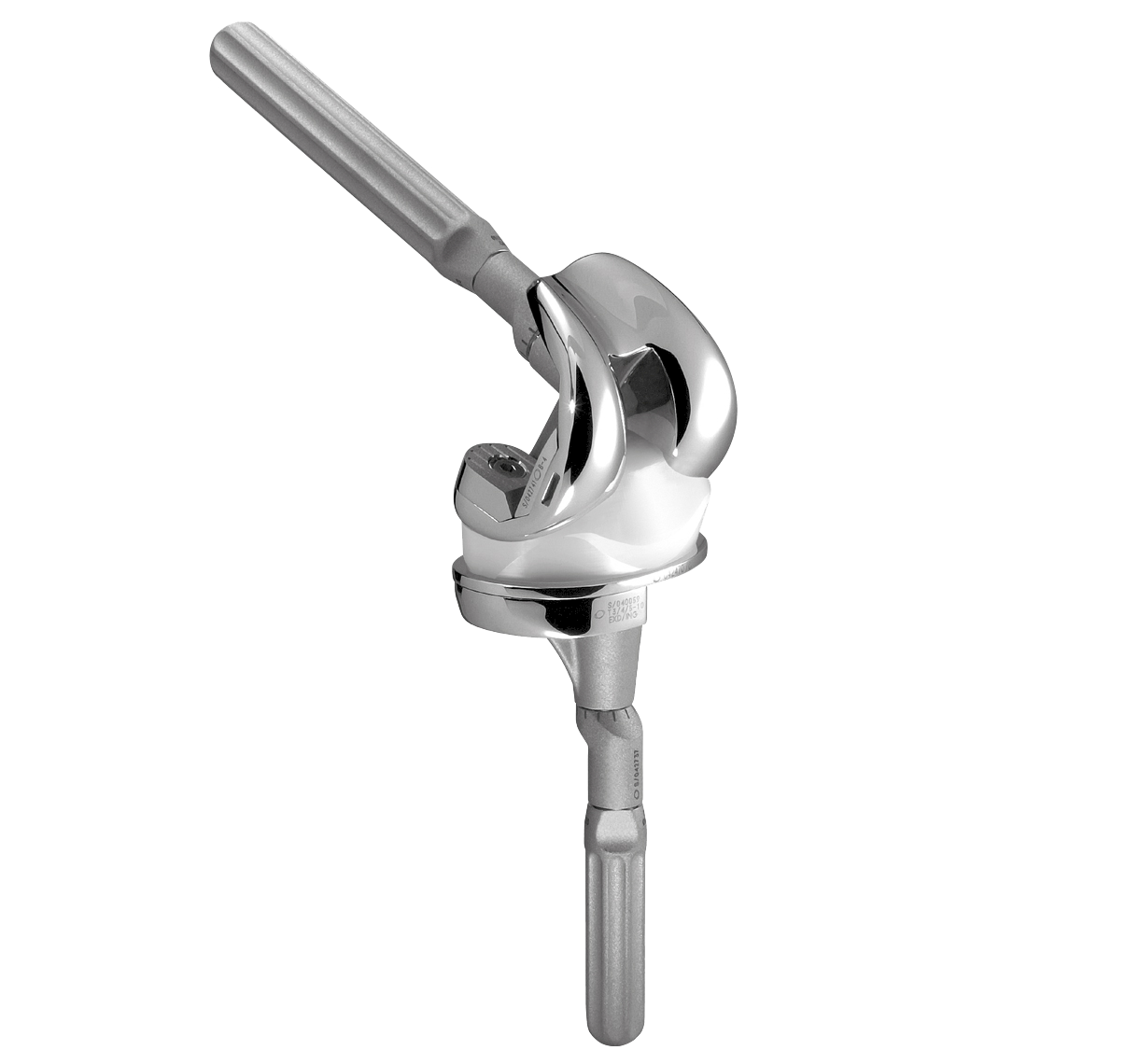
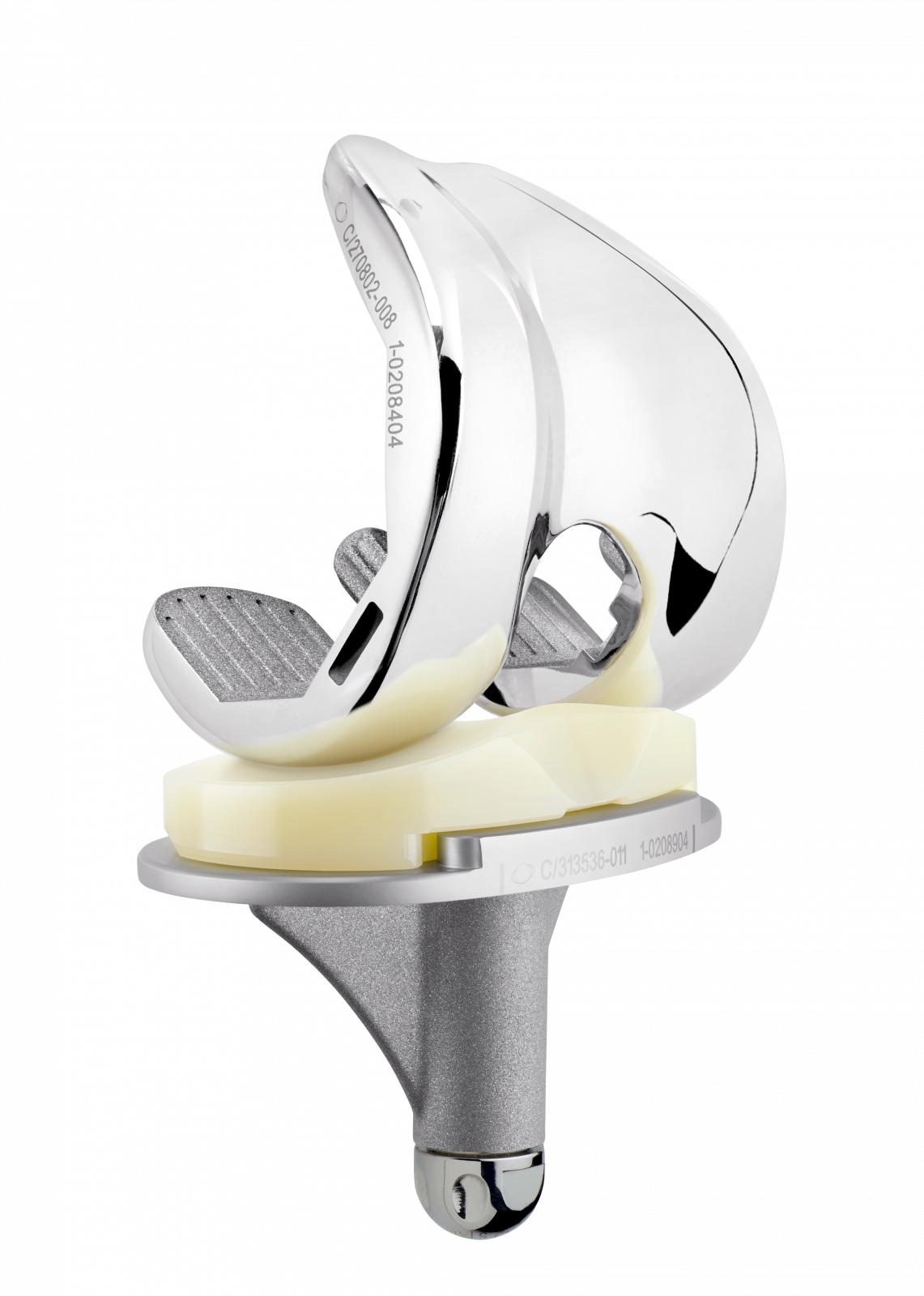
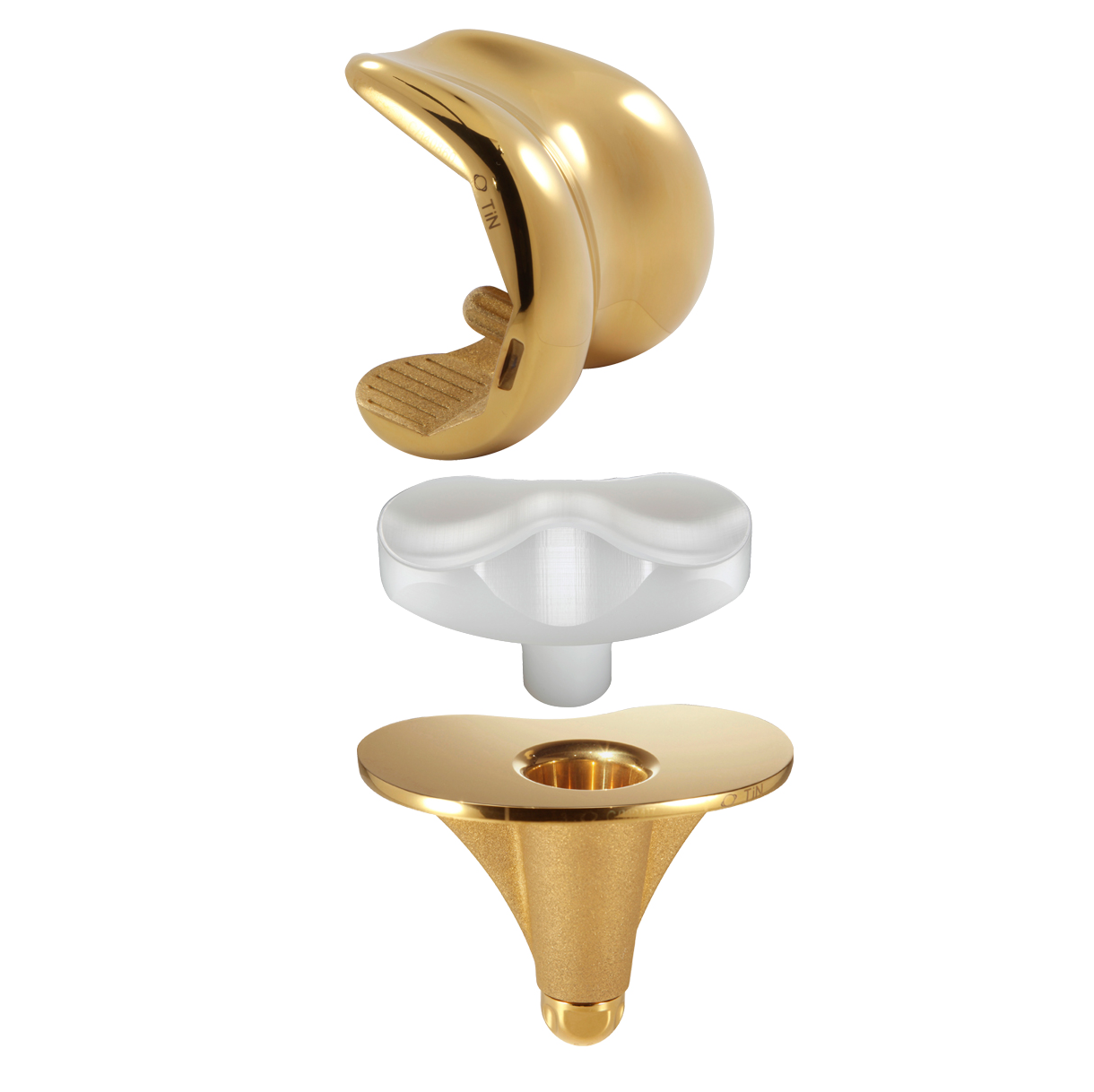
SCORE AS (Allergy Solution) is a primary total knee arthroplasty implant with the same mechanical features as the SCORE TKA implant.
Stability through congruency
Rotating platform, mobile-bearing
PCL-sacrificing
Titanium nitride (TiN) coating
“Some devices may not be approved in your country, please contact your local distributor for further information”
The SCORE AS (Allergy Solution) has the same mechanical features as the SCORE TKA implant.
The 4 µm thick titanium nitride (TiN) layer will prevent release of up to 90% of sensitising metal ions. TiN is four times harder than standard cobalt-chrome and has excellent biocompatibility.
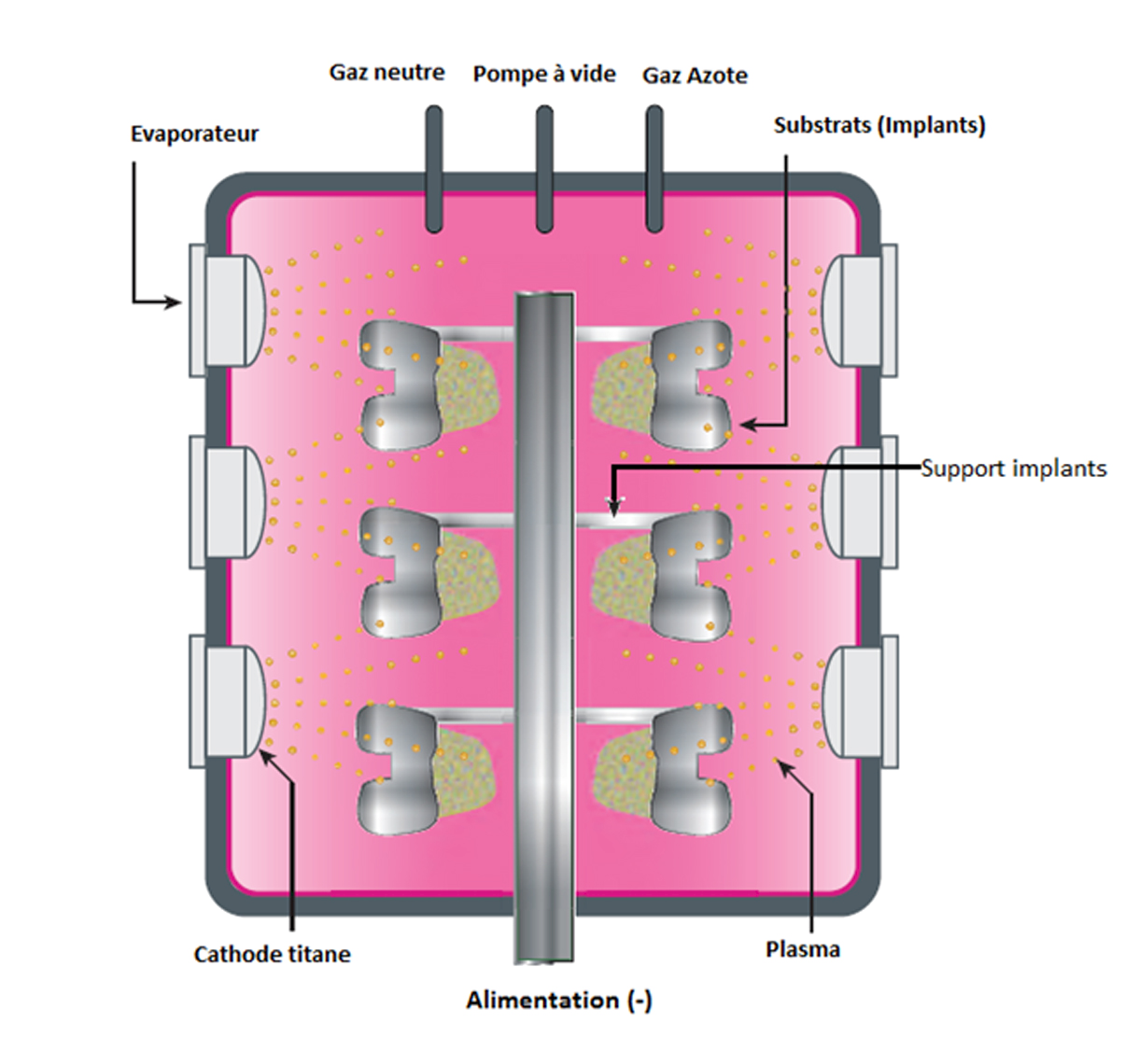
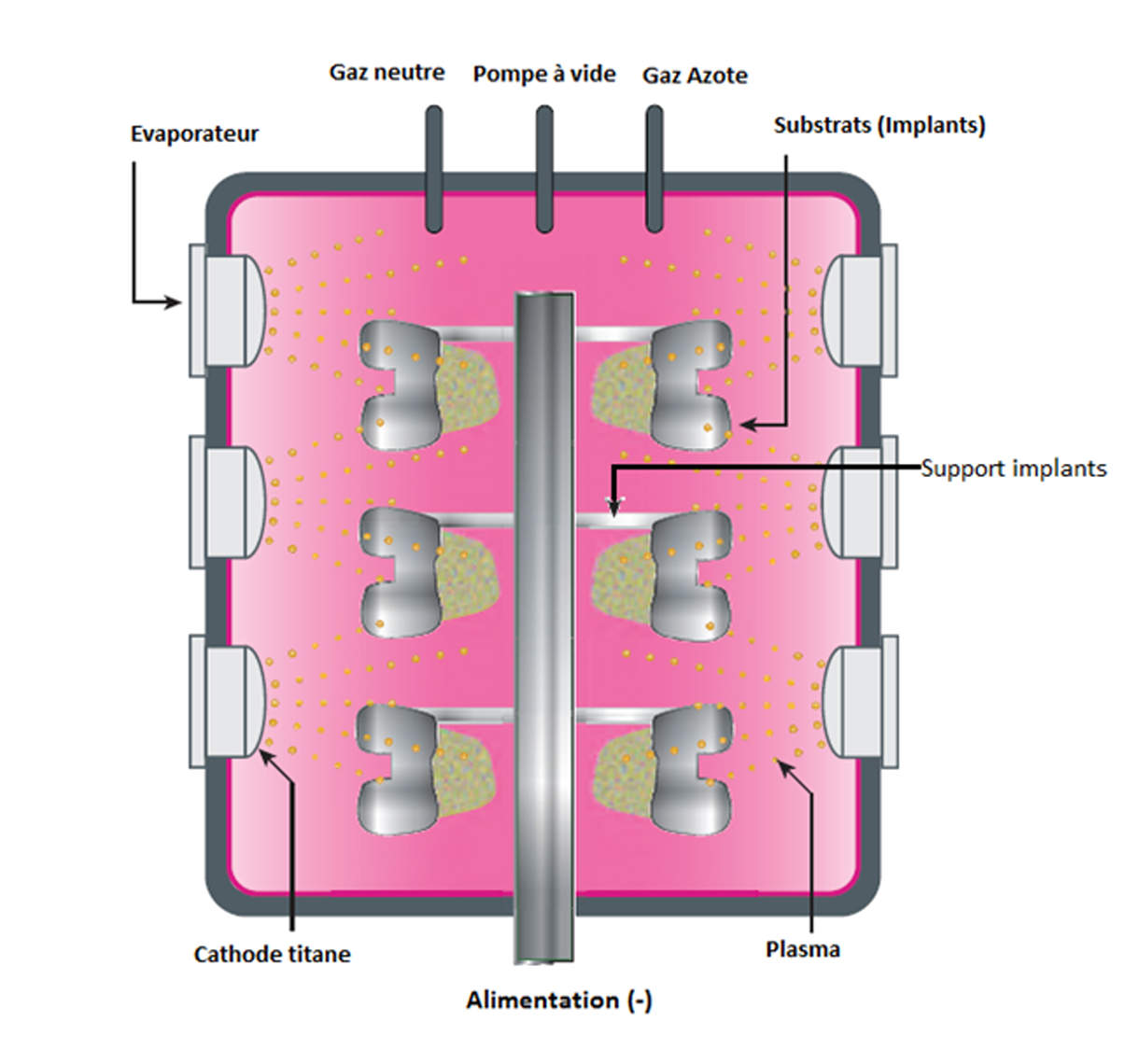
The 4 µm TiN coating is applied by physical vapour deposition (PVD).
Electric arc material (Ti) vaporised from a target (cathode)
Vaporised Ti condenses on the implant surface (anode), resulting in the formation of a thin layer
Nitrogen gas introduced into chamber binds to the Ti
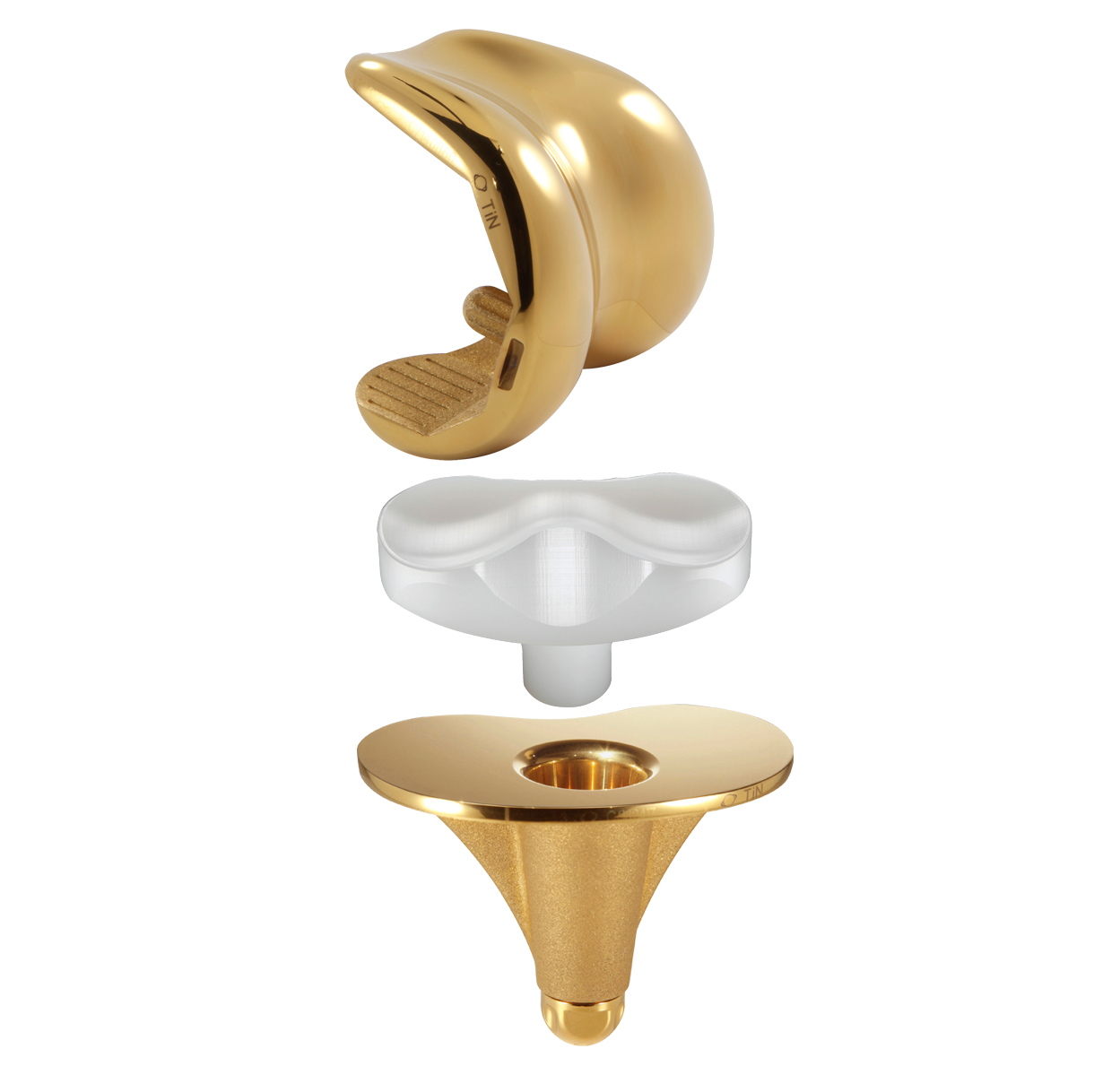
Femoral components:
Cemented: 7 sizes
AP difference between sizes: 2.6 mm
ML difference between sizes: 3.3 mm
Tibial components:
Cemented: 7 sizes
AP difference between sizes: 2.3 mm
ML difference between sizes: 3.5 mm
Inserts:
7 sizes and 5 thicknesses: 10, 12, 14, 16 and 20 mm
 Patellas:
Patellas:
Onset patellar implant - cemented: Ø 30, 33, 36 mm
Inset patellar implant - cemented: Ø 23, 26, 29 mm
Inset patellar implant - cementless: Ø 23, 26, 29 mm
Compatible long keels:
Ø10 to 16 mm
Lengths 75 to 200 mm
Tibial half-wedges
5 mm thickness
10 mm thickness
15 mm thickness
Offset connectors:
2 mm
4 mm
6 mm
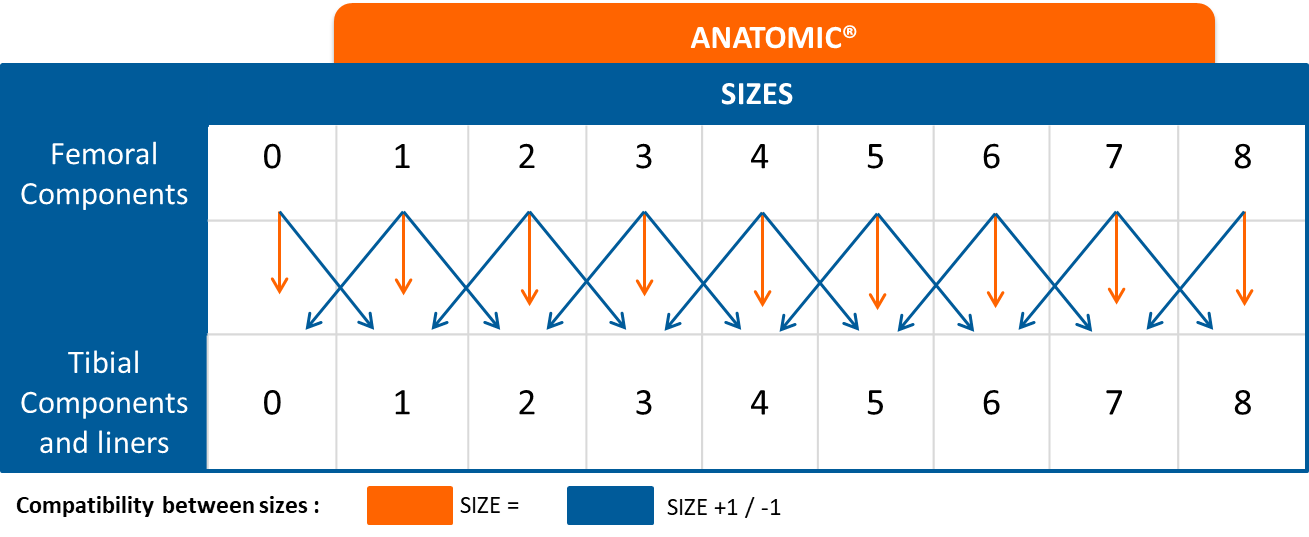
Compatibility between sizes: